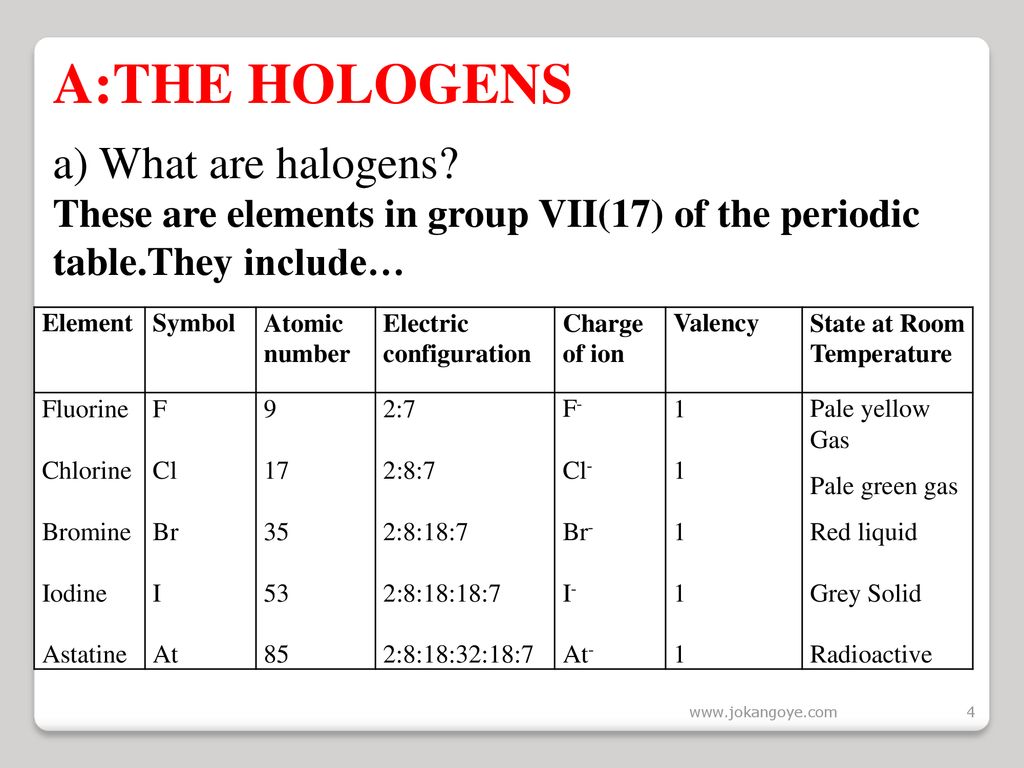
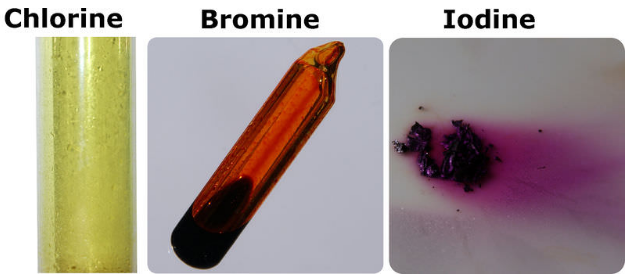
2 9 recall the colours and physical states of the elements at room temperature chlorine gas pale green bromine liquid red brown evaporates to form brown gas iodine solid black sublimes to form purple gas 2 10 make predictions about the properties of other halogens in this group reactivity decreases down the group color darkens down the group.
Chlorine form at room temperature.
Chlorine is a naturally occurring element found in gaseous form at room temperature.
Chlorine has a pungent irritating odor similar to bleach that is detectable at low concentrations.
Chlorine chlorine physical and chemical properties.
It becomes a liquid at 34 c 29 f.
Since it combines directly with nearly every element chlorine is never found free in nature.
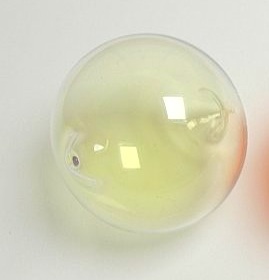
Chlorine is a yellow green gas at room temperature.
Chlorine is a greenish yellow gas at room temperature and atmospheric pressure.
A strong oxidizing agent chlorine deactivates microorganisms by breaking through the cell membrane.
The second lightest of the halogens it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them.
Chlorine is a chemical element with the symbol cl and atomic number 17.
At room temperature chlorine is a yellow green gas that is heavier than air and has a strong irritating odor.
It is two and a half times heavier than air.
It has a choking smell and inhalation causes suffocation constriction of the chest tightness in the throat and after severe exposure edema filling with fluid.
Relative atomic mass the mass of an atom relative to that of.
The temperature at which the liquid gas phase change occurs.
Chlorine is mainly used as bleach in the manufacture of paper and cloth and to make a wide variety of products.
The highly reactive nature of chlorine means it is usually bound with other elements such as sodium chloride salt.
Among the elements it has the highest electron.
Chlorine was first produced by carl wilhelm scheele a swedish chemist when he combined the mineral pyrolusite mno 2 with hydrochloric acid hcl in 1774 although scheele thought the gas produced in his experiment contained oxygen sir humphry davy proved in 1810 that it was actually a distinct.
It can be converted to a liquid under pressure or cold temperatures.
The density of chlorine gas is approximately 2 5 times greater than air which will cause it to initially remain near the ground in areas with little air movement.
Chlorine is a yellow green gas at room temperature.